WHO and Medicines Patent Pool's Sublicensing Agreement: A Game-Changer for Rapid Diagnostics in Low-Income Countries
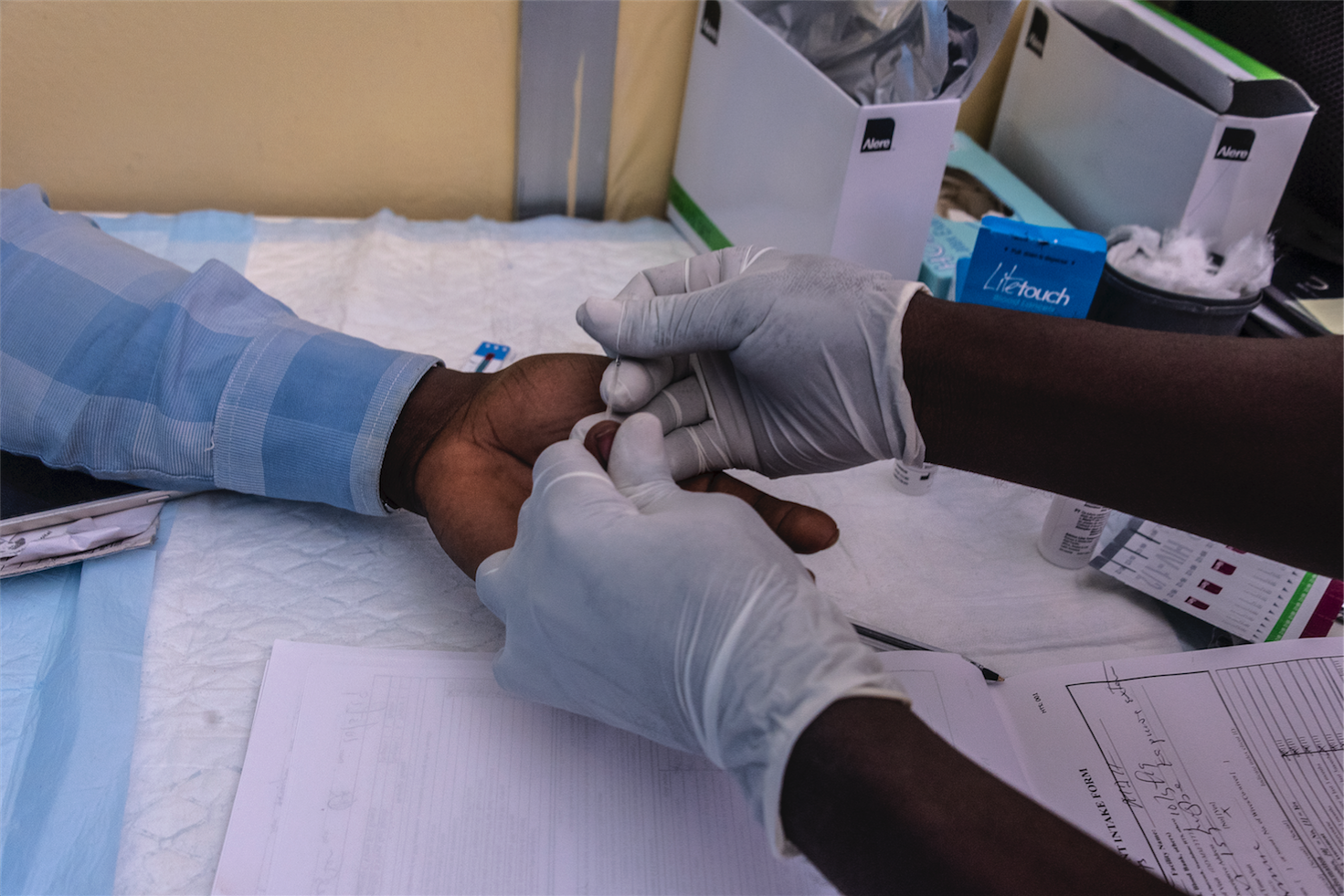
In a world where healthcare disparities are a daily challenge, there’s finally some good news: the World Health Organization (WHO) and the Medicines Patent Pool (MPP) have come together to make essential diagnostic tools more accessible, especially in low- and middle-income countries (LMICs). The announcement on May 9, 2025, about a sublicensing agreement with Codix Bio is a huge leap toward better healthcare accessibility. But how exactly does this affect us? Let’s break it down.
What Does This Agreement Mean for You?
Honestly, the thought of getting a rapid diagnostic test (RDT) within minutes is something most of us take for granted. But for millions in developing nations, this kind of access has been out of reach. Thanks to this new partnership, Codix Bio—a Nigerian health tech company—will be using advanced technology transferred from SD Biosensor to manufacture RDTs locally. The kicker? These tests are simple to use and produce results in just 20 minutes.
The Technology: A Revolution for Healthcare Access
Here’s the thing: rapid diagnostics are about to change the healthcare game. Imagine a world where you don’t need to wait days for a diagnosis or travel to far-off hospitals for testing. The RDTs being produced won’t require any additional equipment, making them perfect for remote areas with limited resources.
And the best part? These tests are incredibly sensitive, meaning you’ll get accurate results faster. Initially, Codix Bio will focus on testing for HIV, but the goal is to extend this to diseases like malaria and syphilis. Talk about a game-changer in public health!
Why Is This Important?
The COVID-19 pandemic showed us just how vital rapid diagnostics are during health emergencies. Whether it's tracking an outbreak or simply getting a quick diagnosis in a rural clinic, this technology can save lives and prevent the spread of diseases. This agreement is part of WHO’s broader initiative to build local manufacturing capacity in LMICs, reducing reliance on expensive imports.
Real-Life Impact: Local Production for Local Needs
Consider this: in many African countries, people often travel long distances to access basic healthcare services. Now, thanks to the partnership between WHO, MPP, and Codix Bio, the power to test and diagnose will be in the hands of local producers. This is exactly the kind of shift we need—local solutions for local challenges.
What’s Next?
The success of this agreement will have ripple effects, especially in future health crises. By enabling local production, WHO is laying the groundwork for self-sustaining healthcare systems. And let’s not forget the potential for scaling these efforts to other critical diagnostics.
FAQ: What You Need to Know
1. What diseases will the RDTs test for?
A. Initially, they’ll focus on HIV, but the plan is to expand to other diseases like malaria and syphilis.
2. How fast are the results?
A. Results will be available in just 20 minutes, making it much quicker than traditional diagnostic methods.
3. Why is local production important?
A. It ensures faster, more affordable access to tests without relying on international imports, which can be expensive and slow.
Conclusion
This new sublicensing agreement is a step forward in improving healthcare equity. It shows that with the right partnerships and technologies, we can bridge the gap in healthcare access and make a significant difference, especially in LMICs.


